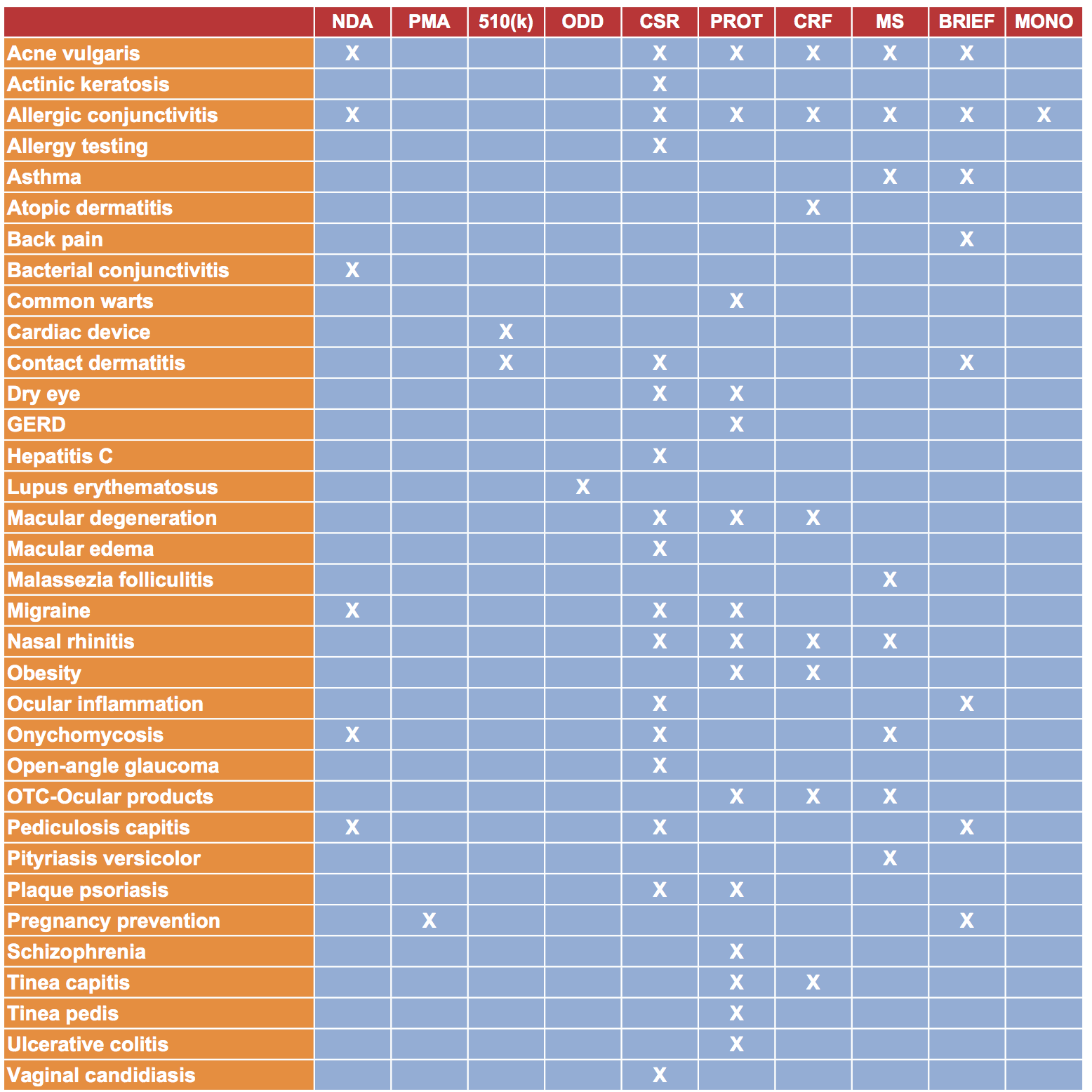
Illuminated Research has experience producing various types of regulatory and scientific documents as indicated below. Within the table, multiple projects may have been conducted within each category (eg, more than one NDA has been prepared for drugs indicated for the treatment of acne vulgaris).
Abbreviations: 501(k) = pre-market notification; BRIEF = regulatory briefing packet; CRF = case report form; CSR = clinical study report;
GERD = gastro-esophageal reflux disease; MONO = product monograph; MS = manuscript; NDA = new drug application;
ODD = orphan drug designation; OTC = over the counter; PMA = pre-market approval; PROT = protocol
GERD = gastro-esophageal reflux disease; MONO = product monograph; MS = manuscript; NDA = new drug application;
ODD = orphan drug designation; OTC = over the counter; PMA = pre-market approval; PROT = protocol